| Monkey Insulin Like Growth Factor Binding Protein 3 ELISA kit |
| Catalog #: E09I0074 |
| Sample Type: Biological samples |
Other Names
BP53; IBP3; Growth Hormone-Dependent Binding Protein; Acid Stable Subunit Of The 140 K IGF Complex
Research Area
Cell Biology, Cancer, Cardiovascular, Metabolism, Signal Transduction
Background
Insulin-like growth factor-binding protein 3, also known as IGFBP-3, is a protein that in humans is encoded by the IGFBP3 gene. IGFBP-3 is one of six IGF binding proteins (IGFBP-1 to IGFBP-6) that have highly conserved structures and bind the insulin-like growth factors IGF-1 and IGF-2 with high affinity. IGFBP-7, sometimes inappropriately included in this family, shares neither the conserved structural features nor the high IGF affinity. IGFBP-3 was first isolated, characterized, and quantitated in human plasma, in 1986.[3][4] It has well-documented functions in the circulation, in the extracellular environment, and inside cells. It is the main IGF transport protein in the bloodstream, where it carries the growth factors predominantly in stable complexes that contain the binding protein, either IGF-1 or IGF-2, and a third protein called the acid-labile subunit or ALS. For IGFs to reach the tissues from the bloodstream, the circulating complexes are believed to partly dissociate, possibly enhanced by limited proteolysis of IGFBP-3. The IGF-1/IGFBP-3 ratio has sometimes been used as an index of IGF bioavailability in the human circulation, but this ignores IGF-1 binding to other IGFBPs (so the ratio is affected by the concentrations of all six IGFBPs), and the fact that IGF-2, which is three times more abundant than IGF-1 in the bloodstream of adults, occupies the majority of binding sites on circulating IGFBP-3. Within tissues, IGFBP-3 can bind IGF-1 and IGF-2 released by many cell types, and block their access to the IGF-1 receptor (IGF1R), which is activated by both IGFs. IGFBP-3 also interacts with cell-surface proteins, affecting cell signaling from outside the cell or after internalization, and also enters the cell nucleus where it binds to nuclear hormone receptors and other ligands. High levels of IGFBP-3 within tumors are associated with increased cancer severity (or worse outcome) for some cancers, but decreased severity or better outcome for others. No cases of IGFBP3 gene deletion in humans have been reported, but mice lacking the gene show near-normal growth. Based on cell growth experiments, animal cancer models, and epidemiological studies, it appears that IGFBP-3 functions as a low-penetrance tumor suppressor gene. Dysregulation of IGFBP-3 has been implicated in many cancers.IGFBP-3 is sometimes referred to as a tumor suppressor, and downregulation of its tissue expression by promoter hypermethylation in some cancers, such as hepatoma. and non-small cell lung cancer may be associated with poor patient outcome. However, consistent with the dual inhibitory and stimulatory roles of IGFBP-3 seen in cell culture, there are other cancer types, such as breast cancer,pancreatic cancer,and clear cell renal cell cancer in which high tissue IGFBP-3 expression has been linked to poor prognostic features or patient outcome. The mechanisms regulating these contrasting effects of IGFBP-3 in vivo are not well understood. Since IGFBP-3 is abundant in the bloodstream of healthy adults (typically 2-4 mg/L), and is largely stabilized by its complex formation with IGFs and ALS, it is unlikely that tumor-derived IGFBP-3 has a large influence on circulating levels. There have been many studies linking circulating IGFBP-3 levels to the presence, or risk, of various cancers, or to patient outcomes.but unequivocal conclusions have often been lacking. For example, high plasma IGFBP-3 levels were associated with a reduced prospective risk of colorectal cancer in women.but in a study including men and women, colon cancer risk was positively associated with plasma IGFBP-3, while there was no significant association for rectal cancer.A large systematic review concluded that circulating IGFBP-3 levels showed a modest association with increased risk for a number of cancers, but the results vary among sites. IGFBP-3 protein levels decrease during the progression of prostate cancer from benign to metastatic disease although production of the protein does not cease completely. IGFBP-3 is still made (at a lower level) by prostate cancer cells and secreted into the surrounding environment. However, instead of the full length, functional protein, IGFBP-3 is found to be cleaved.This decreases the affinity of IGF binding to IGFBP-3, making the growth factors more likely to bind the IGF1R and promote cell survival.
|
Product Name |
IGFBP3 ELISA |
|
Species |
Monkey |
|
Product Size |
96/48 Tests |
|
Concentration |
5-100ng/mL |
|
Sensitivity |
0.48 ng/ml |
|
Principle |
Competitive ELISA |
|
Sample Volume |
100 ul |
|
Assay Time |
90 minutes |
|
Platform |
Microplate Reader |
|
Conjugate |
HRP |
|
Detection Method |
Colorimetric |
|
Storage |
2-8°C |
|
|
For Research use only
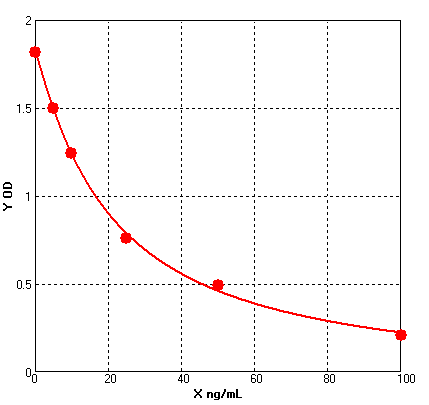
Formular: y = (A - D) / [1 + (x/C)^B] + D
A = 1.82045 B = 1.09299 C = 20.91281 D = -0.06677 r^2 = 0.99895 |
1. Protocols for ELISA
![]() 1) Direct ELISA
1) Direct ELISA
![]() 2) Direct ELISA Using Fluorescent Substrate
2) Direct ELISA Using Fluorescent Substrate
![]() 3) Indirect ELISA
3) Indirect ELISA
![]() 4) Sandwich ELISA
4) Sandwich ELISA
2. Protocols for IHC ICC
![]() 1) Determining if the antibody binds only phosphorylated protein (WB or IHC)
1) Determining if the antibody binds only phosphorylated protein (WB or IHC)
![]() 2) Double immunofluorescence-sequential protocol
2) Double immunofluorescence-sequential protocol
![]() 3) Double immunofluorescence-simultaneous protocol
3) Double immunofluorescence-simultaneous protocol
![]() 4) Fixation and Permeabilization In IHC ICC
4) Fixation and Permeabilization In IHC ICC
![]() 5) Glycol Methalacrylate Acrylic Resin Embedding For IHC
5) Glycol Methalacrylate Acrylic Resin Embedding For IHC
![]() 9) Immunohistochemistry (IHC-Fr) - Frozen Sections
9) Immunohistochemistry (IHC-Fr) - Frozen Sections
3. Protocols for WB
![]() 4) S-100 Mitochondrial Fractionation
4) S-100 Mitochondrial Fractionation
![]() 5) Stripping for Reprobing Western Blots
5) Stripping for Reprobing Western Blots
![]() 7) Western Blotting - A Beginner's Guide
7) Western Blotting - A Beginner's Guide
![]() 8) Western Blotting of Phospho-Proteins
8) Western Blotting of Phospho-Proteins
![]() 9) Western Blotting Using Antibodies Against Histone Proteins
9) Western Blotting Using Antibodies Against Histone Proteins
4. Protocols for IP
![]() 2) Using IgM antibodies for IP
2) Using IgM antibodies for IP
5. Protocols for FACS
![]() 1) Direct Staining Protocol (Cell Surface Staining)
1) Direct Staining Protocol (Cell Surface Staining)
![]() 3) Flow Cytometry Whole Blood Samples-Red Blood Cell Lysis
3) Flow Cytometry Whole Blood Samples-Red Blood Cell Lysis
![]() 4) Indirect Staining Protocol (Cell Surface Staining)
4) Indirect Staining Protocol (Cell Surface Staining)
![]() 6) Recommended Controls for FACS
6) Recommended Controls for FACS
6. Protocols for ELISPOT
![]() 1) ELISPOT
1) ELISPOT
1. Do I have to run all of my standards and samples in duplicate?
Yes, the duplicates are run in order to monitor assay precision and increase confidence in the assay results obtained.
2. Do I have to run all of my samples at one time?
No, each kit uses stripwell microplate. This allows the user to analyse different numbers of samples at different times.
3. What types of reproducible results are obtained with the assays?
Each kit comes with a manual containing a graph of typical data obtained. Any variation in operator, pipetting and washing technique, incubation time or temperature, and kit age can cause variation in result. Each user should obtain their own standard curve.
4. Is it possible to store the reagents other than indicated?
Storage of the kit components under conditions other than indicated is not recommended in order to assure proper performance of the test.
5. How should I store my samples?
Samples should be stored at -20oC or lower temperature. For long-term storage, it is recommended to freeze them at -70oC -80oC.
6. Can I modify the protocol?
BG ELISA kits have been optimized to provide the best possible results. Modifying the format or protocol may give inaccurate and wrong results.
7. Can I use a sample type that is not recommended in the kit insert?
The kit has been validated for the sample types listed in the kit insert. Sample types other than those validated have not been tested. Contact Technical Service for further information.
8. My samples generated values that were outside the dynamic range of the assay. Can I use these values?
It is recommended that only sample values that fall within the range of the standard curve be used. Values outside the range of the standard curve are generally non-linear, which can lead to incorrectly extrapolated values. Samples that generate values higher than the highest standard should be (further) diluted and the assay repeated. If samples fall below the range of the assay, the sample is considered to be non-detectable.
9. Do I have to run a Blank or Zero Standards every time?
Yes, these are required for the calculations, and reflect any subtle but significant performance changes from day to day and assay to assay. They are also extremely helpful when troubleshooting the source of a particular assay problem.
10. Can I alter the volume of sample I use in the assay?
It is not recommended that you alter the volumes since all BG kits are designed for optimal performance at the given volumes
11. Can components from different kits be used?
Each kit contains components which have specific lot numbers to ensure that all of the components are performing optimally alone, as well as with all of the other components in the kit. QC testing is performed on these specific lots. It is never recommended to use your own components or components from other kits or vendors.
12. My standard curve looked fine, but I didn’t get a signal in my sample when I expected to, why?
The sample may not contain the analyte. A matrix effect may be masking the detection. Ensure that the recommended dilution was followed as stated in the kit insert. If dilution was recommended, check to be sure that the dilution was performed properly. Over-dilution may cause the sample to fall below the range of the standard curve.
13. How do you recommend I wash my plate?
If you are using an automated plate washer we recommend that the calibration be checked on a regular basis, and that the system is flushed with the Plate Washing Buffer prior to washing. The same is true for a manual washer. A repeater or a wash bottle can also be used. The user should be careful to ensure that all of the contents are aspirated and the plate tapped dry on lint-free paper.
14. Do I need to use a plate shaker?
Reliable results can be obtained without a plate shaker, but the O.D.'s will generally be lower than those obtained using a plate shaker.
15. Why do I have to use wavelength correction between 450-570nm?
For the ELISA assay, reading at dual wavelengths is done to correct for the optical density contributed by the plastic well, the lamp and optical fluctuations.
16. If I extract my sample, do I still need to follow the recommended dilutions given in the kit insert?
The amount of sample dilution needed after an extraction procedure will be affected by the effects of purification and concentration in the protocol used. The amount of dilution or concentration will have to be determined by the end-user.
17. What is the expected concentration of analyte that I should expect to find?
The amount of a given analyte may vary not only from species-to-species, but also between tissue and cellular sources. The best source of this information is the current literature that is easily accessed through the Internet at multiple scientific databases.
18. My optical densities were a little higher (or lower) than those in the manual that came with my kit. Why?
The optical density is affected by a number of physical conditions such as time and temperature. We suggest that you shorten or lengthen the final incubation with substrate solution to compensate.
19. What are the reasons for High Background?
1) Improper Washing: Check volume of washing buffer reservoir and make sure all recommended washing steps are performed.
2) Contaminated Substrate: Make sure there is no contamination of the substrate with metal ions or oxidizing reagents, before use. Keep the extra substrate solution separately during the ELISA substrate development time.
3) Substrate exposed to light: Exposure to light may result in a blue color of the substrate. Keep solutions in the dark (vial) until ready to dispense into the plate.
4) Wrong Incubation Times/Temperatures: Generally follow the test protocol regarding incubation times and temperatures. However, if all wells are intensely and equally colored with no intensity gradient observed in the standard dilution series, then it may be necessary to observe the substrate reaction as the color is developing, in order to stop the reaction sooner.

 SDS
SDS